How does luminescent paint work?
All you need to know about the principle of photoluminescence
LuminoKrom® glow-in-the dark technology harnesses the natural phenomenon of photoluminescence and the renewable energy of solar brightest radiation.
This physical process enables photoluminescent paint to recharge indefinitely in daylight or artificial lighting, without tiring.
Luminescent paint captures and stores ambient light during the day and releases it in the dark in the form of diffused luminosity for ten hours, without consuming electricity or emitting CO2.
But how does phosphorescent paint actually work?
Luminescence, a natural phenomenon
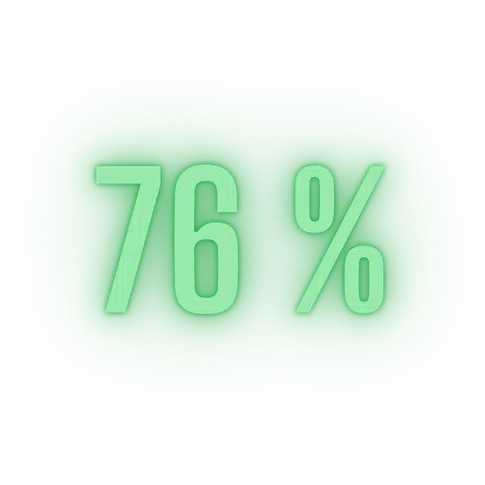
In the ocean, 76% of animals exploit the phenomenon of luminescence. Many fish have developed photoluminescence capabilities for a variety of reasons, including communication, camouflage, attack or defense. They produce their own green-color bioluminescent light, or harbor bacteria that take on this task themselves. Marine creatures use bioluminescence for orientation, communication, but also to view the environment and make the invisible visible...
From bioluminescence to roadside photoluminescence
Following the example of marine animals, we have exploited the exceptional photoluminescent performance of those pigments in our LuminoKrom® paint. The aim is to take advantage of the natural luminosity available in glow materials to enhance the safety of night-time travel (pedestrians, cyclists, etc.) in many local authorities and/or industrial and private sites.
As soon as night falls, LuminoKrom® paint begin to bright in the dark, creating a luminous guide for better orientation and visibility.
Photoluminescence
Photoluminescence results from quick excitation by photon absorption. It's a phenomenon whereby a material absorbs light and re-emits it after a certain delay. Examples include luminescent green or yellow tubes, fluorescent dyes and interior water-based or exterior phosphorescent paints.
Electroluminescence
The electroluminescence results from reactive electrical excitation (electric field), used in light-emitting diodes (LEDs), components used, for example, to backlight LED televisions.
Cathodoluminescence
Cathodoluminescence corresponds to excitation by reactive electron collision. The collision is caused by accelerating electrons using electric fields, for example in cathode ray tubes.
Chimiluminescence
Chemiluminescence is the phenomenon due to an excitation resulting from a chemical reaction, used for example in glow sticks or produced by the blue diffusion flame of a gas burner or lighter.
Bioluminescence
Bioluminescence is an enzymatic reaction, used by fireflies and many marine animals in the water. We could take the example of transparent fish with the skeleton that we can view due to different luminous colors : yellow, black, orange or green.
Crystalloluminescence
Crystalloluminescence results from excitation following a crystalline structural modification, creating and/or breaking chemical bonds in a solid. These are known as mechaluminescence, piezoluminescence and triboluminescence.
Radioluminescence
Radioluminescence is one of the form of luminescence phenomenon resulting from the excitation of X-ray irradiation or α, β, γ radiation, for example in the case of common X-ray screens. It is the radioactive decay of a body.
Scientific advances
1500s - Initial discovery of the phenomenon
The first record of photoluminescence dates back to 1602, when the Italian alchemist and physician Vincenzo Casciarolo discovered that a certain type of stone, called "baryte" (or "Bologna stone"), glowed in the dark after being heated. This stone contained barite, a barium sulfate mineral, which emitted a glow after being exposed to light and heated. This phenomenon intrigued scientists of the time, as it seemed to contradict existing knowledge about light.
1600s-1700s - The studies and research era
Photoluminescence has remained a curiosity for several centuries, observed only sporadically. Researchers such as Robert Boyle and Robert Hooke studied various luminescent materials in the 17th century, but scientific understanding of the phenomenon remained limited.
1800s - Major advances in photoluminescence
In the 19th century, advances in physics led to a better understanding of light phenomena. Scientists like Sir George Stokes, who discovered the phenomenon of fluorescence in 1852, contributed to the study of photoluminescence. Stokes showed that certain materials could absorb ultraviolet light and re-emit visible light, a behavior that laid the foundations for modern understanding of photoluminescence.
"Although fluorescence and phosphorescence, two forms of photoluminescence, have been observed since the 16th century, it wasn't until the early 20th century that we understood the origin of this curious "cold" emission of light."
Bernard Valeur - Pour la science - 2011.
What causes photoluminescence?
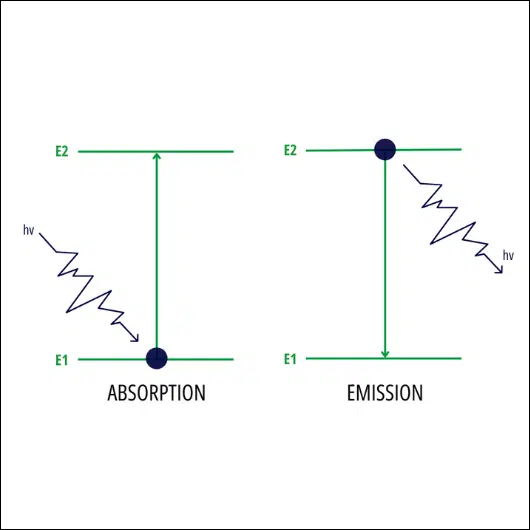
In quantum mechanics, the photoluminescent mechanism involves two different options. The first involves the electronic transition from the ground state to the excited state after absorption of a photon. The second is the return process: the excited state returns to the ground state, spontaneously releasing photons.
Generally speaking, photoluminescence is an optical phenomenon in which materials absorb luminous source at a certain wavelength and emit visible luminous source at a different wavelength. This process generally occurs when electrons in the atoms or molecules of a material absorb light energy and shift to higher energy levels. When these electrons return to their original energy levels, they emit quick luminosity in the form of photons.
What's the difference between fluorescent paint and phosphorescent paint?
In fact, the photoluminescent phenomenon combines the two physical processes of fluorescence and phosphorescence.
- Fluorescence is a photoluminescence that disappears rapidly from 10-9 to 10-6 seconds after quick excitation is extinguished.
- Phosphorescence is a slow photoluminescence from 10-3 to over 10-hour performance when deactivation processes are optimized.
Fluorescence: immediate but temporary luminosity
In this process, electrons absorb energy and move to high reactive energy levels. They will quickly fall back to their original energy levels, emitting light in a very short time, usually in the nanosecond to microsecond range.
Lire la suite
In other words, fluorescence is an immediate and temporary luminous phenomenon. When a fluorescent material is exposed to light, it absorbs this energy and rapidly emits visible light. This relaxation process is virtually instantaneous, and ceases as soon as the light source is removed. As a result, fluorescence is characterized by instant but ephemeral brilliance, fading as the excitation source fades.
Fluorescence is frequently used in our daily lives and in many products. There is a wide choice of "fluorescent" objects, mainly in blue or green color: glow markers, decorative accessories for children, colored aerosol cans, fluorescent-colored clothing, stickers for children, paint, varnish...
Réduire
Phosphorescence, a long-lasting source of diffuse glow in the dark
Phosphorescence is similar to fluorescence, but in this context, the électrons will remain in excited states for a longer period before returning to their original energy levels and emitting light. This delay can range from microseconds to several hours, or even longer.
Lire la suite
The applications of this chemical process are now widely deployed in the field of decoration and furnishing products and resins. There's a wide choice of phosphorescent objects available: pink, black or orange body paint, colored transparent varnish or resin with phosphorescence, phosphorescent ink, easy-to-apply adhesives and stickers, photoluminescent spray cans, accessories and ceiling stars for children. Their luminosity lasts for a a long time after excitation of this type of decorative application.
Réduire
LuminoKrom® photoluminescent paint has been specially designed to exploit the effect of phosphorescence, thanks to its special pigments, in response to safety concerns.
The mechanisms involved in the relaxation process have been optimized to maintain the phosphorescent state in a long-lasting paint, for over 10 hours. A fluorescent paint would have only emitted for fractions of a second.
Our luminescent technology reveals all its magic in the dark, for night-time mobility. The beaconing lights up in the night environment, creating a guide to follow for better orientation.


our brochure
Discover technical details and application tips for our products in the official LuminoKrom® brochure.
"*" indicates required fields
Luminescence, a long-lasting and tireless phenomenon
Phosphorescent paint, product with unlimited luminosity effects
Phosphorescence offers a significant advantage in many everyday contexts. Unlike ordinary paint, phosphorescent paint contains pigments, we choose especially, that absorb natural or artificial bright and then emit a visible glow in the dark.
This ability to store and emit light enables glow paint to serve as an effective means of orientation and reassurance, particularly in areas where lighting is limited. For example, painting objects such as emergency exits, handrails or stair treads with phosphorescent paint will make them easy to spot in the dark, reducing the risk of tripping or accident.
What's more, the quality of our paints is due to the quality of the glow pigments we use, which positively affects the duration and intensity of luminescence.
Find out more about the benefits of using our glow paint for your glow-in-the-dark signage options.
Foolproof robustness
One of the interesting aspects of photoluminescence is its robustness. The electronic change involved in the excitation and relaxation process at the molecular level is extremely small. This explains why the luminescence phenomenon can be reproduced indefinitely without any reduction in luminous performance. The luminance effects in the dark remain intact day after day, night after night.
Studies carried out on markings made on cycle paths using LuminoKrom® photoluminescent paint have shown that luminous properties are maintained for over 5 years, with no deterioration during winter/summer cycles, humidity, snow, etc. A 10% reduction in glow in the dark performance was observed, due to soiling of the marking (dry or wet content, rainy climate...) and not to the quality of our paints.
Exploiting a natural, invisible glow phenomenon
Another fascinating aspect of the photoluminescent process is the presence of emission without any electrical input options. The phenomenon of photoluminescence and/or phosphorescence is a natural process that occurs as soon as a light source is present, whether daylight or artificial lighting. This stored luminous energy is released in the dark, for up to 10 hours.
With LuminoKrom® range, we have demonstrated that the photoluminescent process is independent of the weather. Charging (the excitation process) is just as complete on a rainy day as it is in fog and dry weather. And there's no need for direct sunlight. Excitation and luminous performance are guaranteed even in the middle of a forest, under the shade of trees.
A wide range of photoluminescent options (quart and color)
Our glow-in-the-dark paint catalog offers a range of colors to choose from, providing additional customization and options to suit aesthetic preferences and the specific needs of each situation.
You can find in our catalog different color and quart according to your project. Thanks to online product data sheet and MSDS, you can explore the different options with the quick view and choose the perfect glow marking.
Each color has its own characteristics and luminance performance: white and yellow markings are class E, almond green paint is class G, with 10-hour bright in the dark. Refer to each painting MSDS document and content.
Discover our white marking product sheet and its MSDS document
In short, glow paint offers a practical and versatile solution for improving visibility in the dark, thanks to its unique quick luminous energy storage and emission properties.
Easy to use and various application options
Each of our paints is unique not only in terms of colors, but also in terms of its options of application. Depending on the context of use, different tips may be recommended for optimal and clear application. Each product (available in different quart) in stock is accompanied by its own technical data sheet (MSDS) and clear applicator memo. Our photoluminescent marking solution can be applied with brushes or rollers for indoor use, and with an airless or spray machine for outdoor applications. The most important thing is to respect the dry environnement of application.
Find all our tips for painting with our markings
What's more, our paints, whether solvent- or water-based, are ready to use and require no second coat or protective transparent or invisible varnish.